Urolithin A shows effective against muscular dystrophy
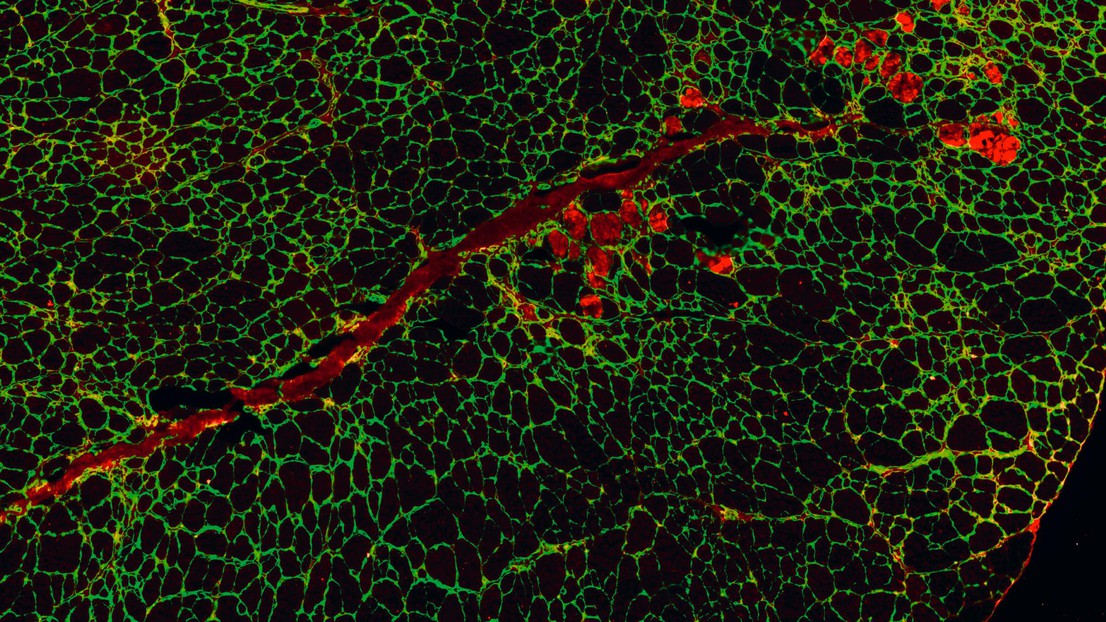
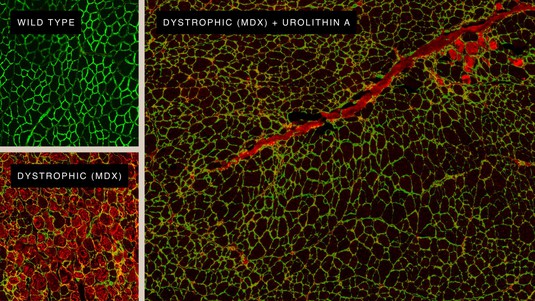
Regenerating muscle cells thanks to Urolithin A. © Amazentis / EPFL Auwerx Lab
A new study published in Science Translational Medicine by EPFL professor Johan Auwerx and scientists from EPFL start-up Amazentis highlights the effectiveness of mitophagy-stimulating molecule Urolithin A in mice to cure a disease similar to Duchenne Muscular Dystrophy. And points to a possible treatment for affected people.
Progression of Duchenne Muscular Dystrophy (DMD) can be delayed in mice by supplementing their diets with Urolithin A, according to new results reported today. The findings, published in Science Translational Medicine, raise hopes that new treatment options could one day be developed for DMD, an uncurable genetic condition characterized by progressive muscle degeneration. About 1 in 3,500 boys are born with DMD, which usually develops in childhood and significantly reduces life expectancy.
The new research carried out at EPFL's Laboratory of Integrative Systems Physiology of Professor Johan Auwerx and the University of Lausanne in collaboration with scientists at the Swiss life science company Amazentis, highlights the important role that defective mitochondria can play in DMD. The powerhouses of cells, mitochondria produce the energy necessary for normal muscle function. But muscle cells taken from both human DMD patients and from mice bred to mimic the condition show significant defects in mitochondrial activity, the study finds. Specifically, patterns of gene expression show the development of DMD is associated with a marked decrease in mitophagy – the process cells rely on to remove and recycle defective mitochondria and maintain energy levels high.
“Duchenne Muscle Dystrophy is the most common fatal genetic disease diagnosed in childhood with still no cure available,” says Johan Auwerx. “Our work represents a significant breakthrough in the search for new therapeutic approaches for muscular dystrophies.”
A powerful natural compound
A natural compound called Urolithin A is known to activate mitophagy in both humans and mice. When the study scientists and lead authors, Peiling Luan and Davide D’Amico, fed the compound to the DMD mice for just ten weeks, they saw mitophagy levels rise effectively, restoring them to normal. This improvement was reflected in drastic reduction of muscle damage and gains in muscle health and performance. The DMD mice administered Urolithin A saw grip strength increase by 31% and running performance increase by 45% compared with control untreated animals. And they lived longer – survival increased by 40%.
Importantly for the human disease, Urolithin A reduced a damaging condition called fibrosis in muscles of the DMD mouse heart and diaphragm, by 36% and 39% respectively. Similar damage seen in DMD patients typically leads to fatal cardiac or respiratory failure. Urolithin A was also able to enhance the regeneration of mouse muscle stem cells. This is particularly relevant to the disease in people as the onset of DMD is linked with the exhaustion of functional stem cells.
Davide D’Amico, PhD, Project Leader at Amazentis and a first author of the paper, said: “Prior to this study, it was understood that the dramatic loss of muscle function in DMD patients was associated with mitochondrial dysfunctions. Here we discovered that mitophagy, the removal and recycling of dysfunctional mitochondria, plays a key role in the progression of DMD when defective.”
Chris Rinsch, PhD, Co-founder and CEO of Amazentis, said, “The rigorous science being published in Science Translational Medicine strengthens the scientific evidence of Urolithin A as a potent muscle function enhancer. It’s exciting to see this natural metabolite can support not only healthy muscle, but also shows promise for progressive muscle diseases in pre-clinical research.”

"Urolithin A improves muscle function by inducing mitophagy in muscular dystrophy", Peiling Luan et. al, Science Translational Medicine, April 7th 2021.