New biologically derived metal-organic framework mimics DNA
iStockPhotos
Chemical engineers at EPFL have synthesized a biologically-derived metal-organic framework on which the hydrogen bonding that forms the DNA double helix can be mimicked and studied like never before.
The field of materials science has become abuzz with “metal-organic frameworks” (MOFs), versatile compounds made up of metal ions connected to organic ligands, thus forming one-, two-, or three-dimensional structures. There is now an ever-growing list of applications for MOF, including separating petrochemicals, detoxing water from heavy metals and fluoride anions, and getting hydrogen or even gold out of it.
But recently, scientists have begun making MOFs, made of building blocks that typically make up biomolecules, e.g. amino acids for proteins or nucleic acids for DNA. Apart from the traditional MOF use in chemical catalysis, these biologically derived MOFs can be also used as models for complex biomolecules that are difficult to isolate and study with other means.
Now, a team of chemical engineers at EPFL Valais Wallis have synthesized a new biologically-derived MOF that can be used as a “nanoreactor” – a place where tiny, otherwise-inaccessible reactions can take place. Led by Kyriakos Stylianou, scientists from the labs of Berend Smit and Lyndon Emsley constructed and analyzed the new MOF with adenine molecules – one of the four nucleobases that make up DNA and RNA.
The reason for this was to mimic the functions of DNA, one of which include hydrogen-bonding interactions between adenine and another nucleobase, thymine. This is a critical step in the formation of the DNA double helix, but it also contributes to the overall folding of both DNA and RNA inside the cell.
Studying their new MOF, the researchers found that thymine molecules diffuse within its pores. Simulating this diffusion, they discovered that thymine molecules were hydrogen-bonded with adenine molecules on the MOF’s cavities, meaning that it was successful in mimicking what happens on DNA.
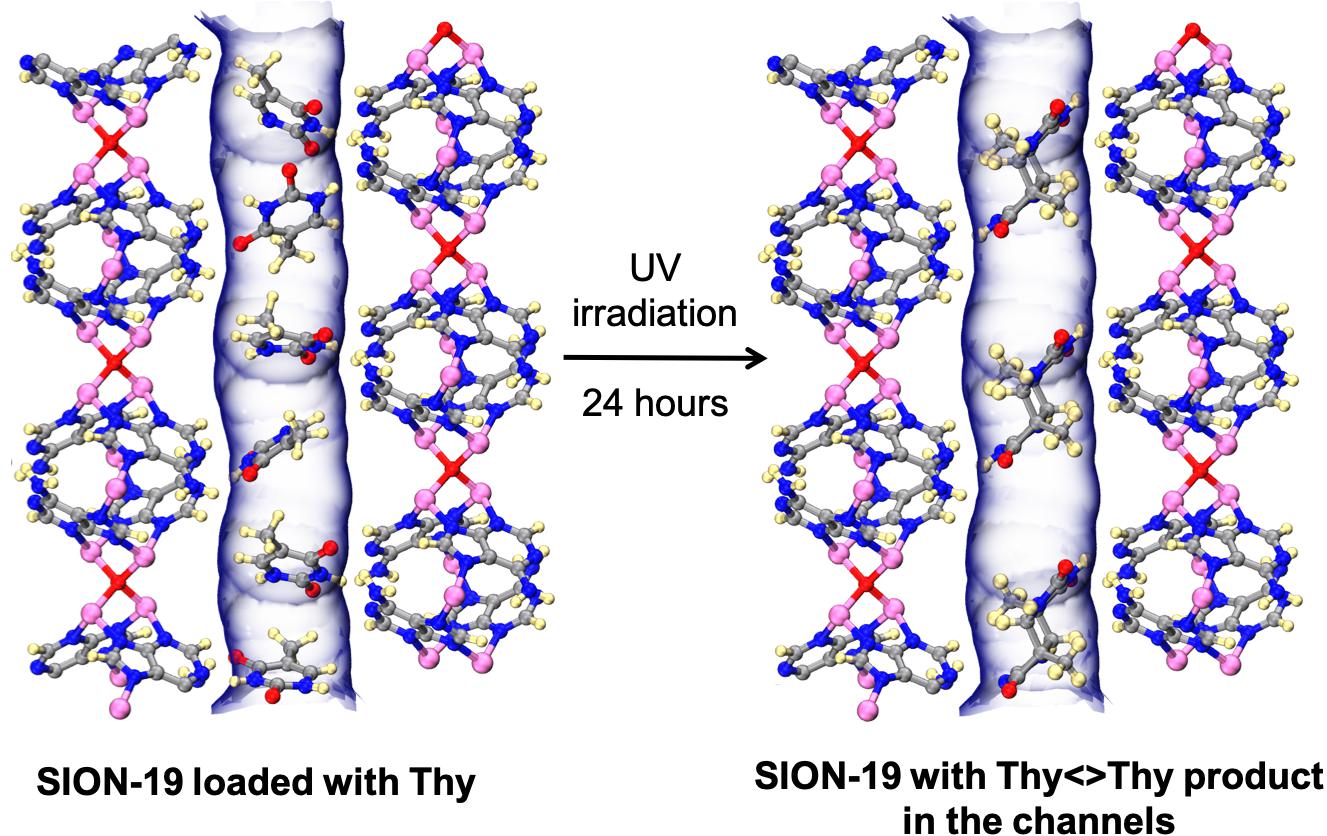
SION-19, a biologically derived MOF based on adenine was used to ‘lock’ Thymine (Thy) molecules in the channels through hydrogen bonding interactions between adenine and thymine. Upon irradiation, thymine molecules were dimerized into di-thymine (Thy<>Thy). Credit: Peter G. Boyd/EPFL.
“The adenine molecules act as structure-directing agents and ‘lock’ thymine molecules in specific positions within the cavities of our MOF,” says Kyriakos Stylianou. So the researchers took advantage of this locking and illuminated the thymine-loaded MOF – a way to catalyze a chemical reaction.
As a result, the thymine molecules could be dimerized into a di-thymine product, which the scientists were able to be isolate – a huge advantage, given that di-thymine is related to skin cancer and can now be easily isolated and studied.
“Overall, our study highlights the utility of biologically derived MOFs as nanoreactors for capturing biological molecules through specific interactions, and for transforming them into other molecules,” says Stylianou.
Other contributors
- University College London
- University of Southampton
- University of Liverpool
Swiss National Science Foundation (Ambizione Energy Grant and NCCR-MARVEL), ERC Advanced Grant, UK’s Engineering and Physical Sciences Research Council (EPSRC), Swiss National Supercomputing Center (CSCS)
Samantha L. Anderson, Peter G. Boyd, Andrzej Gładysiak, Tu N. Nguyen, Robert G. Palgrave, Dominik Kubicki, Lyndon Emsley, Darren Bradshaw, Matthew J. Rosseinsky, Berend Smit, Kyriakos C. Stylianou. Nucleobase pairing and photodimerization in a biologically derived metal-organic framework nanoreactor. Nature Communications 08 April 2019. DOI: 10.1038/s41467-019-09486-2