How bigger molecules can help quantum charge flow last longer
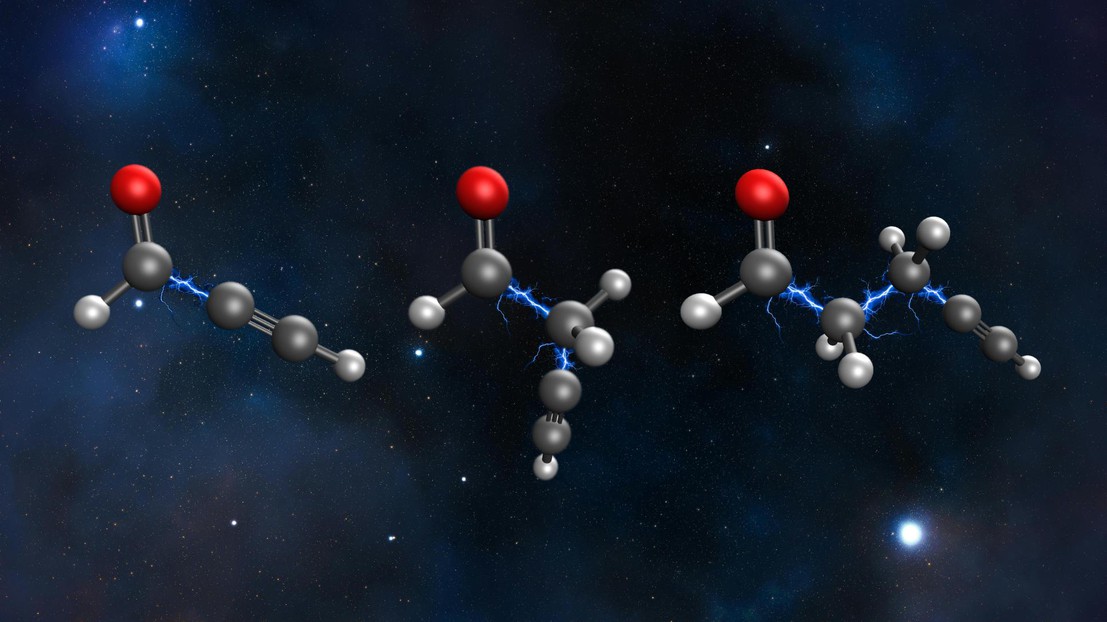
Inserting –CH₂– chemical groups into propynal increases molecular size and flexibility, yet unexpectedly favors the persistence of quantum effects. 2025 EPFL/Alan Scheidegger - CC-BY-SA 4.0
A team at EPFL and the University of Arizona has discovered that making molecules bigger and more flexible can actually extend the life of quantum charge flow, a finding that could help shape the future of quantum technologies and chemical control.
In the emerging field of attochemistry, scientists use laser pulses to trigger and steer electron motion inside molecules. This degree of precision could one day let us design chemicals on demand. Attochemistry could also enable real-time control over how chemical bonds break or form, lead to the creation of highly targeted drugs, develop new materials with tailor-made properties, and improve technologies like solar energy harvesting and quantum computing.
But the big roadblock is decoherence: electrons lose their quantum "sync" within a few femtoseconds (a millionth of a billionth of a second), especially when the molecule is large and floppy. Researchers have tried different methods to sustain coherence—using heavy atoms, freezing temperatures etc. Because quantum coherence vanishes at macroscopic scales, most approaches to sustaining coherence operate on the same assumption: larger and more flexible molecules were assumed to lose coherence more rapidly. What if that assumption is wrong?
Investigating the question, three researchers, Alan Scheidegger and Jiří Vaníček at EPFL, and Nikolay Golubev at the University of Arizona, studied a series of simple organic molecules, each with terminal alkyne and aldehyde groups separated by a chain of carbon atoms. They used simulations to show that making the carbon chain longer actually helped electrons stay in sync for longer. The discovery could help in designing molecules that hold on to their quantum properties longer.
Modeling every tiny motion of atoms and electrons would have been too complex and computationally impossible. So the researchers used a smart shortcut: they treated the atomic nuclei—the heavy cores of atoms—as moving according to the rules of classical mechanics, like tiny billiard balls, yet accounting for their quantum nature in an approximate way, while carefully tracking the lighter electrons using the exact laws of quantum mechanics, fully capturing their wave-like and probabilistic nature. This approach, called semiclassical dynamics, gave them a detailed view of which specific atomic vibrations disrupted the fragile quantum state and which ones allowed it to survive longer.
The study found that adding carbon atoms slows down decoherence. In larger molecules like pentynal, certain vibrations that would normally disrupt the electron flow became much less active or even vanished. In fact, the study showed that only particular vibrations that preserve the molecule's symmetry had a significant impact on coherence. In contrast, out-of-plane vibrations, which might have been expected to cause disruption, turned out to have almost no effect.
The researchers also found that charge migration not only lasted longer but also became easier to observe. When a molecule loses an electron, it leaves behind a "hole"—an area of positive charge that acts like a missing electron. In the largest molecule studied, this migrating hole moved more smoothly and predictably along the carbon chain, with fewer disruptions from internal vibrations. This clearer and more stable movement makes it easier for scientists to precisely time interventions, such as using a second laser pulse to influence chemical reactions. In short, making the molecule bigger helped stabilize charge migration instead of disrupting it.
Quantum coherence isn't just a laboratory phenomenon—it's essential for technologies like quantum computers, ultra-sensitive sensors, and laser-driven chemical control. The study shows that by making molecules larger and more flexible—without losing their chemical reactivity—scientists can actually extend quantum coherence and stabilize charge migration.
“Charge migration is currently a highly active research area and is central to the emerging field of attochemistry, which relies on sustained electronic coherence,” says Alan Scheidegger, a PhD student at EPFL and the lead author of the study. “More broadly, extending coherence times is of significant interest for researchers developing quantum technologies.”
Swiss National Science Foundation (SNSF) through NCCR Molecular Ultrafast Science and Technology (MUST)
European Union’s Horizon 2020 research and innovation program through ERC Consolidator Grant MOLEQULE
U.S. Department of Energy (DOE)
Alan Scheidegger, Nikolay V. Golubev, Jiří J. L. Vaníček. Can increasing the size and flexibility of a molecule reduce decoherence and prolong charge migration? PNAS, 30 May 2025. DOI: 10.1073/pnas.2501319122