Foreign vs own DNA: How an innate immune sensor tells the difference
Scientists at EPFL and the Friedrich Miescher Institute have used cryo-electron microscopy to explain how a DNA-sensing biomolecule that is key to our innate immunity response is inactivated when it comes in contact with the cell’s own DNA.
A biomolecule that gained considerable attention over the past few years is cGAS, a "DNA sensor" that is involved in kickstarting immune responses in the body. Specifically, when a pathogen is infecting a cell, cGAS senses its DNA and begins a cascade of biochemical reactions that activate the body's so-called "innate" immune system – the first-line-of-defense part of our immune system.
One of the main scientific mysteries surrounding cGAS has been how it can distinguish self- from non-self DNA. But even more perplexing is the fact that cGAS also exists inside the cell's nucleus, where all the genetic material is stored.
Now, scientists at EPFL and the Friedrich Miescher Institute in Switzerland have revealed an intriguing mechanism on how nucleosomes – the building units that package DNA inside the nucleus – bind and inactivate cGAS. Published in Nature, the collaborative work was led by EPFL Professor Andrea Ablasser and FMI scientist Nicolas Thomä, and provides new insights into the biology of this immunity-crucial molecule.
A nucleosome consists of a string of DNA wrapped around pairs, or “dimers”, of proteins called histones, like a thread around a spool. The mix of histones and DNA is referred to as “chromatin”.
Previous studies have found that when cells are treated with the anti-cancer drug aclarubicin, certain histones are “mobilized” from chromatin. At the same time, cGAS also becomes mobilized, which made the researchers in this study conclude that cGAS might somehow associate with the nucleosome in the cell. The idea was finally confirmed with biochemical assays that showed that the two can indeed interact.
To further understand this phenomenon, the scientists used cryo-electron microscopy to see, on a structural level, how nucleosomes bind to cGAS. Studying the complex formed by cGAS and nucleosomes at a resolution of 3.1 Ångstroms (0.31 nm), they found that cGAS binds to the so-called “acidic patch” of the nucleosome; a negatively charged platform that commonly binds proteins. Once bound there, both histones and the nucleosome DNA “trap” cGAS in a state in which it is unable to engage or sense DNA, and is therefore functionally inactivated.
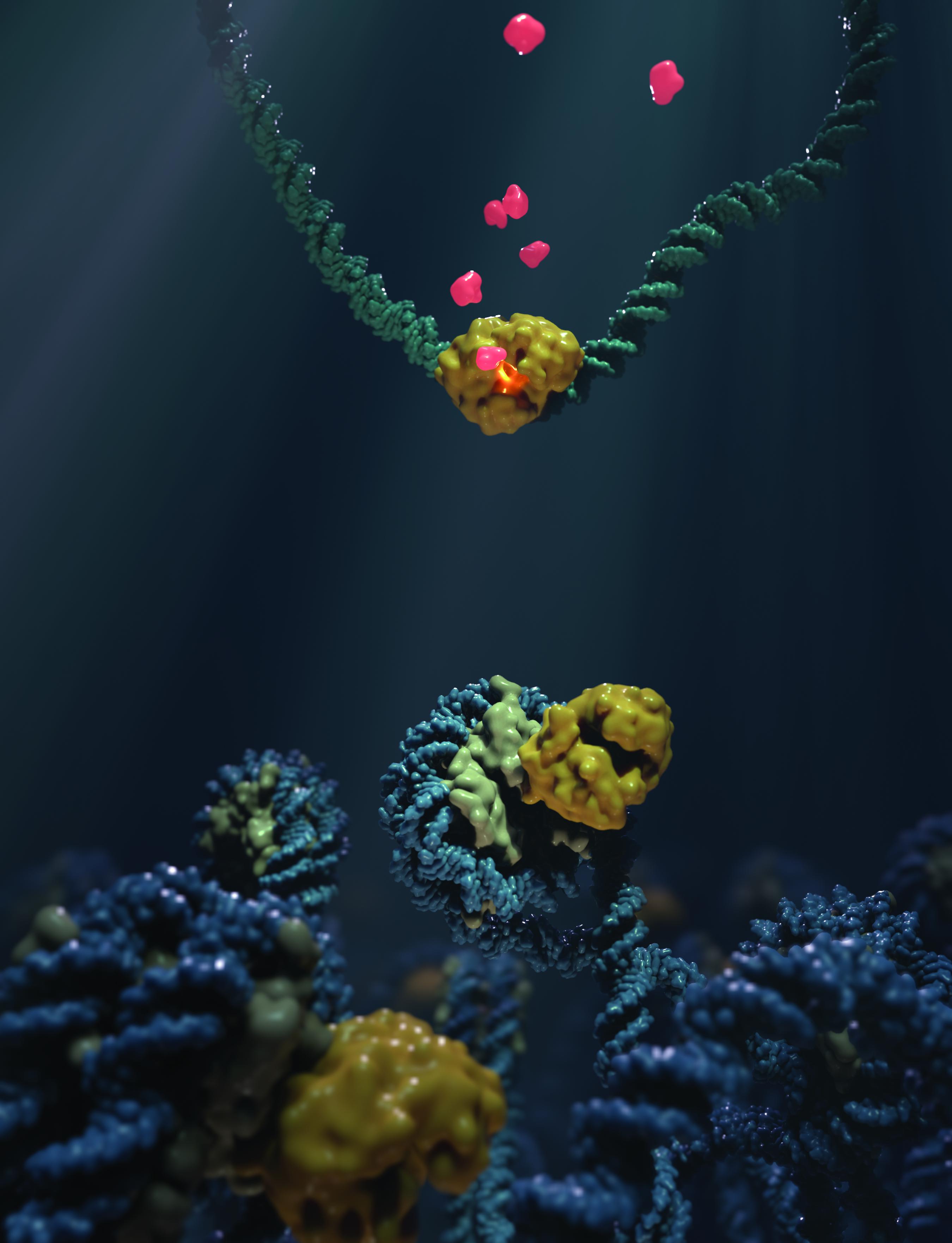
To validate their findings, the scientists introduced mutations to the cGAS-acidic patch interface, which effectively prevented cGAS from binding the nucleosome. This was enough to remove the blocking effect of the nucleosome on cGAS, and unleashed a strong immune response in living cells.
The work offers the first structural clue to how chromatin interferes with the activity of cGAS. It reveals the structural basis of cGAS interaction with chromatin provides scientists with a mechanism of how cGAS can discriminate between its own cell’s DNA and that of foreign pathogens.
Other contributors
- University of Basel
- EPFL Institute of Chemical Sciences and Engineering (ISIC)
Swiss National Science Foundation (SNSF)
NCCR in Chemical Biology
Horizon 2020
Novartis Research Foundation
Ganesh R. Pathare, Alexiane Decout, Selene Glück, Simone Cavadini, Kristina Makasheva, Ruud Hovius, Georg Kempf, Joscha Weiss, Zuzanna Kozicka, Baptiste Guey, Pauline Melenec, Beat Fierz, Nicolas H. Thomä, Andrea Ablasser. Structural mechanism of cGAS inhibition by the nucleosome. Nature (2020). DOI: 10.1038/s41586-020-2750-6